By Huri Mücahit The following blog post was written after attending the iJOBS workshop: Primer in PK/PD held on February 20th, 2019
How exactly do pharmaceutical companies choose which medication to pursue for the treatment or prevention of an illness? The answer is through the study of pharmacology (the analysis of interactions between drugs and the human body), pharmacokinetics (the study of drug movement throughout the body (PK)), and pharmacodynamics (the body’s biological response to the drug (PD)). During the iJOBS PK/PD workshop, Dr. Anson Abraham, Principal Scientist at Merck and Co., provided a deeper look at the science behind these interactions.
Understanding the relationship between medications and the body is fundamental for any treatment, thus, the bulk of the data collected during clinical trials is related to PK/PD analysis. In fact, roughly half of a drug label is informed by these analyses, including the sections covering dosage and administration, dosage forms and strengths, drug interactions, and clinical pharmacology.
[caption id="attachment_2751" align="aligncenter" width="2154"]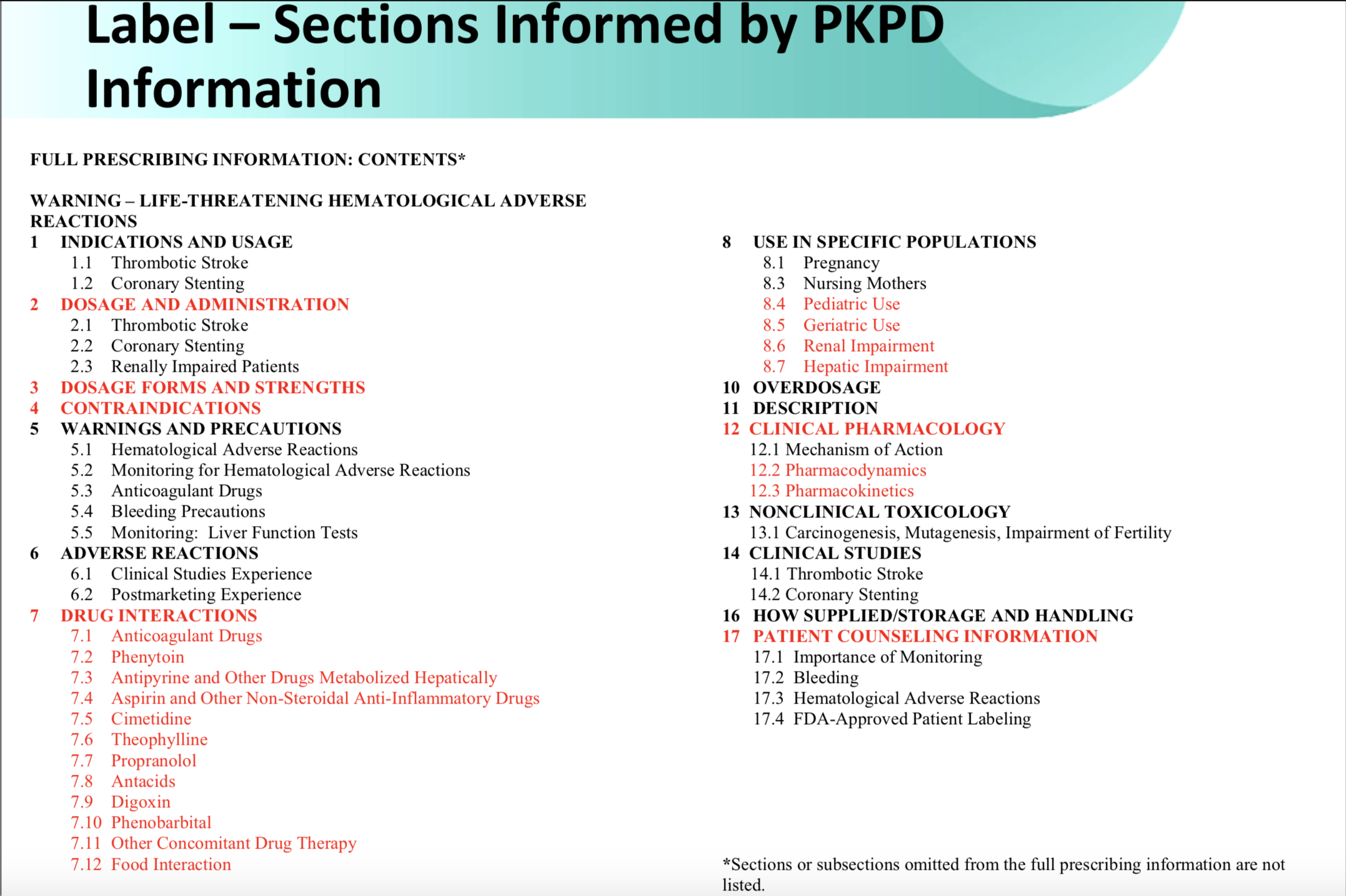 Dr. Anson Abraham, Merck and Co., 2019[/caption]
Dr. Anson Abraham, Merck and Co., 2019[/caption]
In order to collect PK information, researchers look at the processes after drug administration, which are collectively called “ADME” - absorption, distribution, metabolism and elimination of the drug. To start off, a drug can only have an effect if it has been absorbed within the body; therefore, factors such as molecule size and structure, permeability across the gastrointestinal membrane, and the extent and rate of absorption are carefully considered. Following this, it must be determined if the drug reaches its intended site. It is important to identify known binding targets of the drug, or if it is absorbed within specific tissues, as this will impact drug dosage and forms. Researchers then analyze the metabolism of the drug, although this is a greater consideration for small molecules rather than large molecules. The four common types of reactions are: oxidation, hydrolysis, reduction, and conjugation; drug-drug interactions are predicted based on this metabolic profile. Finally, whether the drug can be eliminated must be taken into account, as accumulation within the blood stream can lead to toxicity. Due to the in-depth analyses required, as the image below outlines, this entire process is completed within 10-12 years. As such, PK/PD analysts must not only be patient, but efficient, with extracting the relevant information from large amounts of data.
[caption id="attachment_2750" align="aligncenter" width="660"]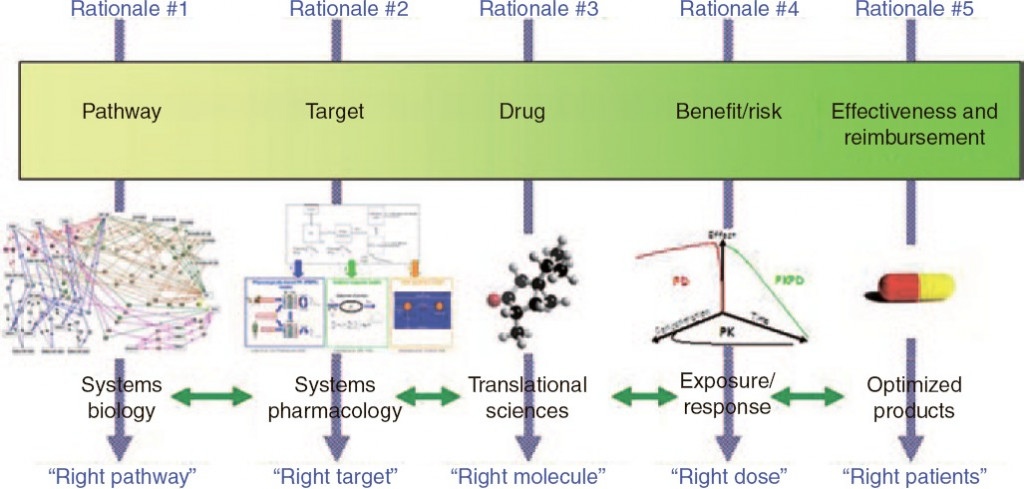 Clinical Pharmacology & Therapeutics
Clinical Pharmacology & Therapeutics
Volume 93, Issue 6, pages 502-514, 14 MAR 2013 DOI: 10.1038/clpt.2013.54[/caption]
If this career sounds appealing to you, Dr. Abraham has several tips. First, as this profession requires an understanding of both the biology and the math behind the analyses, a PhD student in the biological sciences should consider strengthening their mathematical skills. Courses offered at Rutgers, such as Statistics in Clinical Research and Fundamentals in Analysis, and through other specialized programs can help with this endeavor. Additionally, pharmaceutical companies such as Merck often provide internships that include PK/PD work experience. If you cannot commit to a full summer internship during your PhD studies, the iJOBS program aids Phase 2 students in finding externships, which are less time-consuming, but still provide experience within the field. Additionally, these externships provide an opportunity for students to determine whether they would like to work in the lab to collect the relevant data or analyze the data once it has been generated.
If you’re looking for further information on the PK/PD workshop, feel free to visit the iJOBS page for the complete slide deck.
Edited by: Emily Kelly Castro, Monal Mehta, and Paulina Krzyszczyk