This post was written following the iJOBs Workshop: From Bedside to Bench and Back: regulatory requirements for collaborations between pharma industry and academia, on February 27 by Damir Hamamdzic, PhD. Throughout the past decades, expansion of academic-industry partnerships has become a more prominent feature in the broader landscape of partnerships within biomedical innovation. Some of the benefits of this type of scientific collaboration are: (1) pharmaceutical companies are outsourcing their Research and Development (R&D) to academic institutions as an opportunity to cut cost (2) access to groups of experts in the different areas, which represents a competitive advantage for the participating companies; and (3) the desire and the impulse of scientists to participate in entrepreneurial activities. The pharmaceutical regulations put great emphasis on manufacturing their products using practices and processes that ensure high levels of safety and efficacy in every step. These standards ensure the secure and effective products for patients. Dr. Damir Hamamdzic, compliance administrator at the Office of Regulatory Affairs at Rutgers University, explains that though the potential value of academia-industry collaboration is generally apparent, all stakeholders need to recognize that research activities must be conducted in a manner that adheres to the principles of sound scientific methods and ethical requirements while, at the same time, acknowledging the missions and responsibilities of the involved institutions. One way to do this is the academic implementation of Good Clinical Laboratory Practices (GCLP). 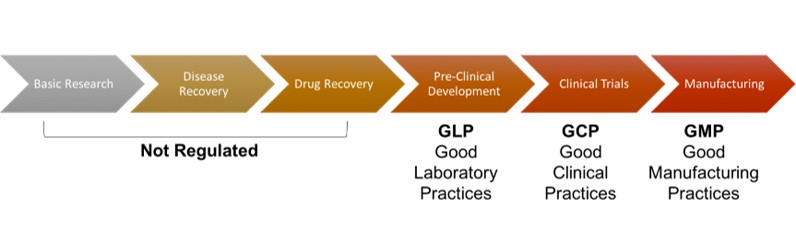 Good Laboratory Practices (GLP) regulations and Good Clinical Practices (GCP) regulations are well established as regulatory requirements for pre-clinical and clinical studies, respectively. However, a remarkable gap exists between collaborating institutions, especially since universities generally do not target FDA submissions when conducting their studies. GLP is designed to protect scientific data integrity, and to provide the regulatory agencies (EPA, FDA) an auditable record of open-ended research studies (OECD 1998). On the other hand, GCP is an international ethical and scientific quality standard for designing, conducting, performing, monitoring, auditing, recording, analyzing and reporting clinical trials that involve the participation of human subjects. GCLP provides a bridge between GLP and GCP, and more importantly ensures the reliability and integrity of data generated in analytical laboratories. It is a framework in which organizations can base to develop facilities, systems and procedures that guarantee the laboratory work and results fulfil the GLP and GCP expectations. The workshop concluded with an interactive activity to identify potential problems on five scenarios. For example, one of the scenarios presented that a laboratory’s protocol stated that their experiments will be performed in accordance to GLP regulations and guidelines. In this case, this statement was wrong because in reality the university, or the specific lab, is not monitored by federal agencies, following the appropriate regulations. Some other general points discussed after analysis and evaluation of these were focus on quality of facilities and instrumentation, proper transportation and storage of materials (ex. plasmids, cell cultures), personal qualifications, certification of protocols and reagents, good recompilation of reports, training records and data backup. There are many opportunities for industry–academia collaborations in different fields -genomics, biomarkers, animal models, for example. Being conscious and understanding the proper requirements and regulations to enhance these partnerships is crucial. Junior Editor: Eileen Oni Senior Editor: Aminat Saliu Musah
Good Laboratory Practices (GLP) regulations and Good Clinical Practices (GCP) regulations are well established as regulatory requirements for pre-clinical and clinical studies, respectively. However, a remarkable gap exists between collaborating institutions, especially since universities generally do not target FDA submissions when conducting their studies. GLP is designed to protect scientific data integrity, and to provide the regulatory agencies (EPA, FDA) an auditable record of open-ended research studies (OECD 1998). On the other hand, GCP is an international ethical and scientific quality standard for designing, conducting, performing, monitoring, auditing, recording, analyzing and reporting clinical trials that involve the participation of human subjects. GCLP provides a bridge between GLP and GCP, and more importantly ensures the reliability and integrity of data generated in analytical laboratories. It is a framework in which organizations can base to develop facilities, systems and procedures that guarantee the laboratory work and results fulfil the GLP and GCP expectations. The workshop concluded with an interactive activity to identify potential problems on five scenarios. For example, one of the scenarios presented that a laboratory’s protocol stated that their experiments will be performed in accordance to GLP regulations and guidelines. In this case, this statement was wrong because in reality the university, or the specific lab, is not monitored by federal agencies, following the appropriate regulations. Some other general points discussed after analysis and evaluation of these were focus on quality of facilities and instrumentation, proper transportation and storage of materials (ex. plasmids, cell cultures), personal qualifications, certification of protocols and reagents, good recompilation of reports, training records and data backup. There are many opportunities for industry–academia collaborations in different fields -genomics, biomarkers, animal models, for example. Being conscious and understanding the proper requirements and regulations to enhance these partnerships is crucial. Junior Editor: Eileen Oni Senior Editor: Aminat Saliu Musah
iJOBS Blog