In the last decade, several reports have been published indicating that there has been a shift in the mindset of students graduating with a degree in biomedical sciences. Now, more than 50 percent of biomedical PhD scientists are seeking non-traditional or non-academic careers (Zimmerman AM 2018; American institute of research 2014).
Medical Affairs has emerged as an attractive non-traditional career for many PhD scientists in recent years. Pharmaceutical companies are always in pursuit of the next drug/target for a disease and to bring that next product into market. In a pharmaceutical company, medical affairs functions as a team connecting external customers with internal teams, such as research and development (R&D). Medical Affairs is also a scientific team that functions alongside R&D in a company.
On November 19th 2018, Rutgers iJOBS hosted a workshop at the Rutgers New Jersey Medical School on to topic of careers in Medical Affairs. This workshop was run by Dr. Paul Weber, currently serving as an Associate Dean for Continuing Medical Education at Robert Wood Johnson Medical School and New Jersey Medical School. Previously, he has held many positions in pharmaceutical companies including Celgene, PTC therapeutics, Roche and Valeant Technology.
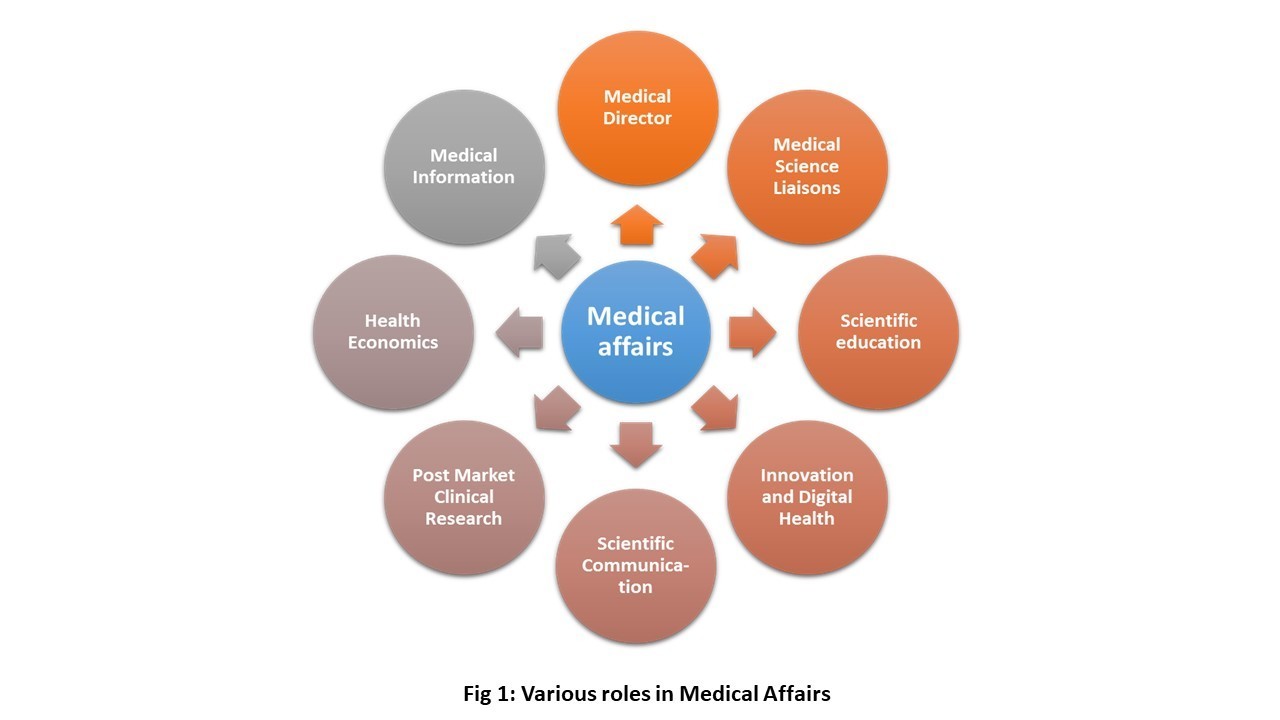
Dr. Weber began the workshop by giving a brief overview of Medical Affairs. During this presentation, he listed the teams that representatives in medical affairs must interact with including R&D, regulatory affairs, marketing, sales and legal. In addition to working with other internal teams, there are also specific roles played by the individuals involved in medical affairs, which are outlined below:
Medical Science Liaisons (MSL): Medical science liaisons act as the connection between internal company stake holders and external key opinion leaders in a therapeutic area. They must have excellent communication and interpersonal skills.
Scientific Education (Continuing Education and Continuing Medical Education): People in this role are responsible for grant support in medical education to healthcare providers.
Innovation and Digital Health: This department mainly deals with developing new applications in order to improve outreach to patients or customers, and also provide them with accurate drug information.
Scientific Communications: The main role of the scientific communication team is to put together internal data from clinical trials for public disclosure while taking into consideration legal and regulatory affairs related the drug.
Medical Director (Domestic/Global): This is the highest position assumed by a person in medical affairs. The medical director oversees all teams in medical affairs, reviews critiques of company products, and is also aware of competitors in the market.
Dr. Weber also explained several other roles in medical affairs such as post market clinical research, health economics and medical information. He then went onto discuss requirements or credentials needed to secure a position in medical affairs. Scientific background is a must with awareness in business, public health and health policy. A certification in medical affairs is a plus. Anyone with an advanced degree such as PhD, PharmD and MD are eligible to apply for positions in medical affairs.
After the overview in medical affairs, we had what is called a “world café experience”. The attendees split into groups and discussed various roles in medical affairs over snacks. One of the most interesting things I learned during group discussion was that students wanted to pursue a career in medical affairs because it would allow them to work as a team and travel. This is quite contrary to the reclusive nature of PhDs in a graduate school program. Medical science liaison was the most attractive career choice for graduate students in medical affairs.
Later, Dr. Paul Weber went on to define medical affairs as a dynamic and rapidly growing area of the pharmaceutical and device industries that focuses on research, knowledge and education. Furthermore, medical affairs provide primary scientific and medical support for a company’s marketed products and development pipeline. He also pointed out advancements that came into existence in the past decade including electronic health records and applications (sensors, wearables and telehealth). Also, he discussed how social media has changed the course of medicine and platforms such as Snapchat, Twitter and Linkedin are in use to market drugs and treatments.
Finally, attendees participated in a medical affairs simulation workshop. There were six drug targets: HER2 Inhibitor, VEGF inhibitor, BRAF inhibitor, CAR-T, PD-1 inhibitor and Alk inhibitor. Each group selected a particular drug target and role-played different team members in medical affairs. After a brief discussion, the medical director in each group outlined the drug target and the responsibilities assumed by each team member to bring the drug into market. Overall, the simulation workshop was an excellent tool to understanding a career in medical affairs and what it might entail in the real world.
Thanks to Rutgers iJOBS for hosting this event and also to Dr. Paul Weber for conducting the workshop. It was a great experience and a refreshing way to learn about a career in medical affairs.
This post was written by Madhuri Bhagavathula. Edits to this post were made by Monal Mehta , Paulina Krzyszczyk and Eileen Oni
REFERENCES:
- Navigating the path to a biomedical science career; Zimmerman AM; PLOS One 2018 Sep 7;13(9):e0203783. doi: 10.1371/journal.pone.0203783. eCollection 2018
- https://www.air.org/news/press-release/sixty-one-percent-stem-phds-pursue-nonacademic-careers