by Sumiyya Raheem
On February 13th, Dr. Joanna Ghayad, a leadership and career coach, led an iJOBS event on the transition from academia to industry and the cross functional teams involved in drug discovery within industry. Dr. Ghayad earned her PharmD from the University of the Sciences in Philadelphia in 2009 and initially worked in the medical field as a clinical oncology pharmacist. Then, she transitioned to industry as a medical science liaison at United Therapeutics Corporation and eventually Bristol Meyers Squibb. In October of 2024, she launched her own leadership and career coaching business, Joanna Ghayad Coaching. This background equipped her with the expertise to effectively lead this seminar.
This seminar provided a broad-spectrum explanation of what transitioning into industry entails. Dr. Ghayad broke it down from multiple perspectives, from academic research versus industry research to how they differ in terms of the type of funding, research freedom, innovation, and work environment. Academic research is primarily focused on advancing knowledge, understanding mechanisms and contributing to scientific literature. In contrast, industry research is more market-driven with an emphasis on developing profitable products. In terms of funding, academic research relies entirely on government and non-profit grants while industry research is funded by various sources, including venture capital firms and other private companies. Government sourced funding and non-profit grants enable academic researchers more freedom which results in more innovative research.
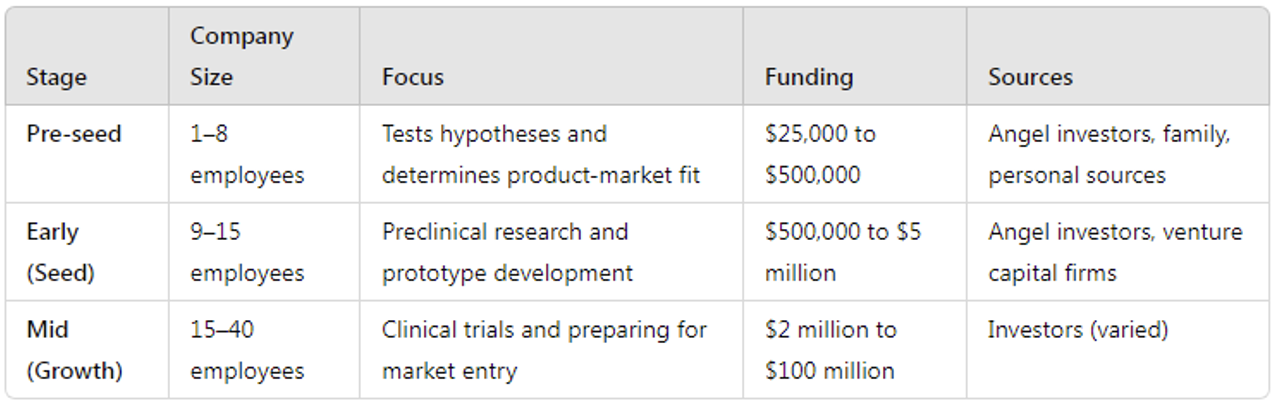
Most academics looking to step outside of academia will create a startup company first. These startup companies provide an excellent opportunity to gain a diverse set of skills quickly by working closely with multiple departments, fundraising, and networking. The skills they gain strengthen their resumes and prepare them for the transition to a larger company. Other academics may never leave academia, maintaining a startup company alongside their laboratory at an academic institution. These startup companies have milestones that mirror the broader development process of an established pharmaceutical company. The three major stages of startup growth are as follows:
One particularly valuable part of the seminar was Dr. Ghayad’s breakdown of pharmaceutical organization reporting structures. She explained the hierarchy of pharmaceutical companies, focusing on the Chief Medical Officer (CMO) and the departments under research and development (R&D) and highlighting roles where scientists can transition into non-benchwork positions. Within R&D, program leadership plays a crucial role in moving a drug from discovery to clinical trials. Many scientists are also now entering medical affairs, which includes fields such as:
- Medical communications – advising pharmaceutical companies on how to educate customers about new therapies and drugs.
- Medical publications – creating and managing materials, such as manuscripts and presentations, to inform patients and healthcare professionals.
- Pharmacovigilance – monitoring and assessing drug safety, including data collection and reporting.
Beyond R&D, scientists are transitioning into business development, working in sales or as consultants who help companies navigate investments. These roles are essential in guiding pharmaceutical companies on which scientific innovations or drugs to pursue.
Toward the end of her talk, Dr. Ghayad provided an excellent overview of the drug development process, from target identification and validation to clinical development. The early stages involve validating biological targets and creating high-level plans for translational research. This is followed by compound screening and lead discovery, where candidate compounds are identified and preliminary tests begin. In the preclinical development stage, candidates are optimized and prepared for clinical supply. Once these phases are completed, the drug progresses to clinical trials (Phase 1) where data is collected for patient stratification.
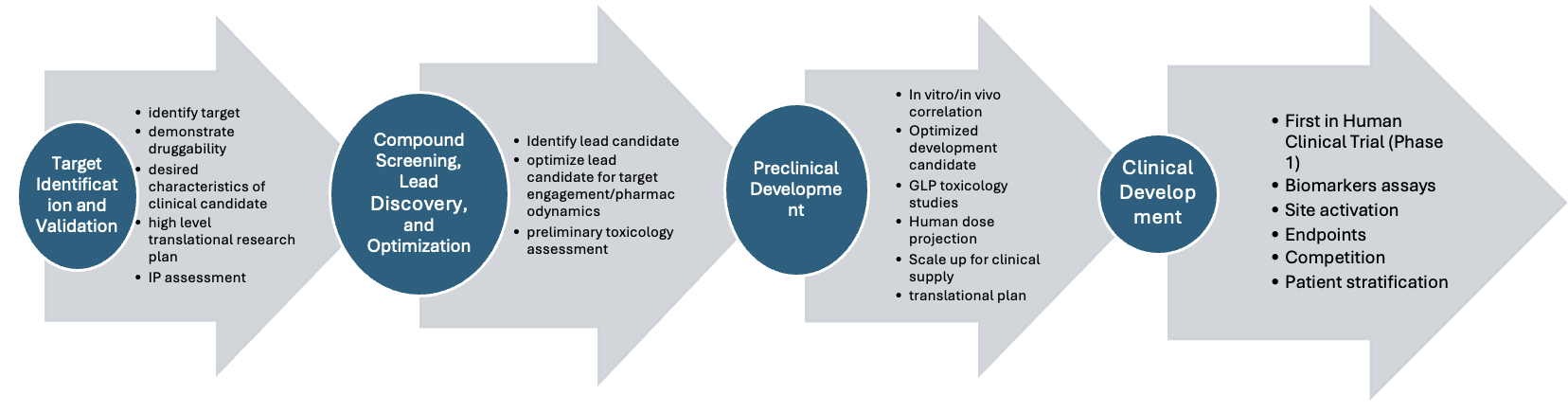
She emphasized that bringing a drug to market requires collaboration across many disciplines. Discovery biology initiates the process, but success depends on a wide range of professionals, including those in medical affairs, business development, and regulatory affairs.
Overall, Dr. Ghayad’s seminar provided both a macro and micro perspective on transitioning into industry, helping attendees determine which sector best fit their skills and interests. Her comprehensive breakdown of the drug discovery process and industry career paths made it clear that there are numerous opportunities to become a successful scientist outside of the traditional academic positions.
This article was edited by Junior Editor E. Beyza Guven and Senior Editor Antonia Kaz.