[caption id="attachment_3184" align="aligncenter" width="660"]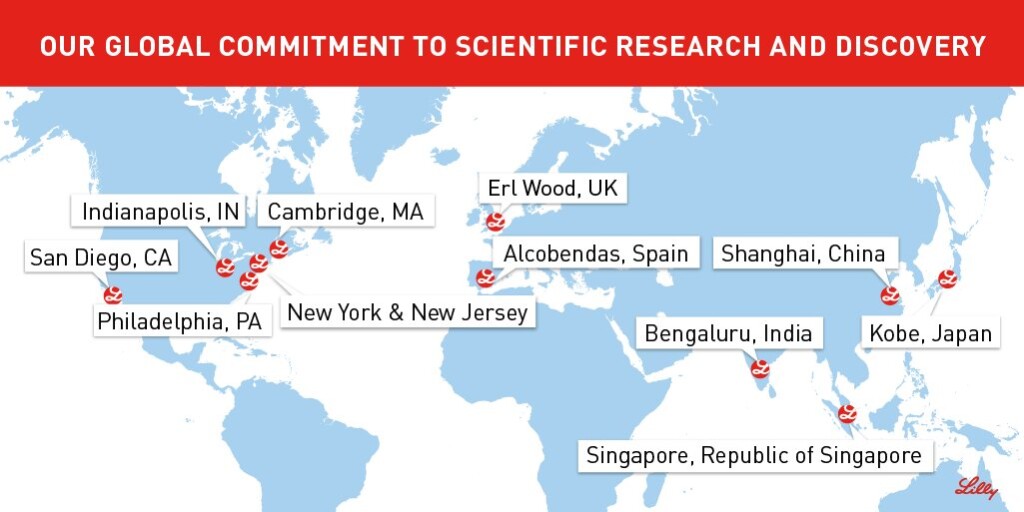 The locations of Eli Lilly's research and development facilities (note that this does not include the company's manufacturing plants or sales offices). From https://twitter.com/LillyPad/status/739118029976506368/photo/
The locations of Eli Lilly's research and development facilities (note that this does not include the company's manufacturing plants or sales offices). From https://twitter.com/LillyPad/status/739118029976506368/photo/
By: Zachary Fritz
Despite a still raging global pandemic, on Friday, June 19th curious graduate students were able to “virtually” step inside one of the world’s major pharmaceutical companies, Eli Lilly, via an iJOBS Virtual Career Panel. Eli Lilly is a Fortune 500 company that has about 34,000 employees worldwide with offices, manufacturing plants, and research and development facilities in 13 countries. The company focuses on developing medicines in the areas of oncology, diabetes, chronic pain (such as migraines, cluster headaches, and pain due to osteoarthritis), autoimmune diseases, and neurodegenerative diseases. Some of Eli Lilly’s best known drugs include Prozac, Cymbalta, Cialis, and Humalog. Instead of actually visiting one of Eli Lilly’s campuses, the event proceeded as a Zoom career panel with four current employees of the company. They represented a diverse cross-section of Eli Lilly, coming from varying backgrounds, with half of them being relatively recent hires (1 year or less at the company). All were able to provide valuable insights into their paths to Eli Lilly, their day-to-day work, the company culture, and what they considered the most valuable skills and qualities for their fields. While the job titles and descriptions of the panelists varied greatly, the common themes of collaboration, time management, and problem solving skills were readily apparent between them. Dr. Anchal Sharma, a postdoc at Eli Lilly’s New York City site, mentioned that she typically juggles 3-4 projects ranging from wet lab-based applications like single-cell RNA sequencing to more computational work like analyzing clinical trials data. Similarly, Dr. Sinem Ozguli, a Senior Principle Research Associate in Biochemistry Quality Control, reinforced the need for proper time management and flexibility in her role, which involves writing and revising technical documents like SOPs and ensuring that manufacturing facilities are complying with regulatory and Good Manufacturing Practices (GMP) requirements. In her six years at the company, Dr. Delise Oyola-Robles emphasized her need for cross-collaboration and understanding between scientists, engineers, and commercialization personnel, especially in her former role as a “molecule steward”, a kind of scientific project manager that oversees all aspects of manufacturing a particular drug. Dr. Justin Gaudet, a consultant process microbiologist, said that he was able to leverage his PhD training in order to “ask the right questions” and solve problems, such as developing strategies to prevent microbial contamination of manufacturing processes. For these reasons the panelists actually emphasized “soft” skills and qualities—like communication, leadership, and critical thinking—over technical, STEM-based skills, particularly when applying for a job at Eli Lilly. While a typical interview will include a presentation of your PhD work to demonstrate your technical expertise, your answers to questions regarding your personality and interpersonal skills will be what really distinguishes you from other applicants. The panelists also advised keeping your resume concise and focused; most likely you should not include every publication, activity, or position you held. Dr. Sharma instead said you should “be specific” and “summarize your work under one umbrella”, giving your resume a cohesive theme or story. While three of the four panelists had postdoc positions prior to joining the company, having one isn’t necessarily a prerequisite to employment at Eli Lilly. Dr. Delise Oyola-Robles was actually hired through her prior position at Bristol Myers Squib, showing that industry experience and networking also goes a long way. If you do end up with a job at Eli Lilly, the panelists noted that you can look forward to excellent employee investment, horizontal mobility, and work-life balance. Dr. Ozguli mentioned that training is a continuous, long-term process, and for those employees looking to advance their knowledge and skills in a particular area the company is often willing to fund trips to conferences or training programs at one of its many locations. It is also not difficult to change positions and locations within the company. Dr. Oyola-Robles’ six year tenure at Eli Lilly is testament to this. She started with Eli Lilly working in Technical Services and Manufacturing Sciences at the Indianapolis headquarters, then moved back to her native home of Puerto Rico to help start up a fermentation pilot plant there, and is now joining the Quality Control department as Senior Responsible Scientist at the Branchburg, NJ site. The panelists also noted that since most of the PhD-level positions involve managing your own time and projects, carving out a good work-life balance is certainly manageable. If you’re interested in working at a company with a myriad of opportunities, locations and a positive impact on global health, consider Eli Lilly! Continue checking out iJOBS events and the iJOBS blog for tips on how to improve your interview, resume, and networking skills to help you get your foot in the door.
Editor: Monal Mehta