Research Opportunities at the FDA
While we eagerly wait for the next round of iJOBS events and the start of the semester, let me give a brief summary of a career panel hosted last May, detailing excellent research careers at the U.S. Food and Drug Administration (FDA). I found out from this career panel that there are as many as around 3000 Ph.D. graduates employed in the FDA! When we consider places to apply to after graduation, we usually think of academia or industry, but somehow overlook the FDA and other government organizations. As fellow blogger Samantha recently discussed, there are several places that offer career opportunities outside both academia and industry, including the FDA. Thanks to iJOBS, and the two FDA panelists, we were given a great inside look at this agency.
Rita Humeniuk, Ph.D., M.Sc. Eng. is currently a Commissioner’s Fellow in the Office of Clinical Pharmacology in the Center for Drug Evaluation and Research where she works on a regulatory research project on pediatric drugs. Her M.Sc. in Biotechnology and Engineering opened her interest in how drugs work, which led to her pursuing a Ph.D. in Pharmacology. She obtained a Fellowship at the National Cancer Institute, which introduced her to the science of drug development, preclinical/translational research, and clinical pharmacology. Following this, she rounded off her training by applying for and obtaining an FDA Commissioner’s Fellowship, which exposed her to regulatory science, public health impact, and drug discovery management. All of these training and experiences, Dr. Humeniuk shared, made her well suited for a job in either the FDA or in industry.
Dr. Humeniuk gave an introduction on the different roles and functions of the FDA. Under the big FDA umbrella are various centers that focus on everything the public consumes and uses, from drugs, vaccines, and medical devices, to food, cosmetics, etc (link to FDA site). She focuses on the Center for Drug Evaluation and Research (CDER), which largely oversees (1) drug development, (2) drug quality throughout its life cycle, and (3) post-marketing safety and promotion. CDER accomplishes these goals through a multi-disciplinary review team to answer the question: Is the drug safe and efficacious?
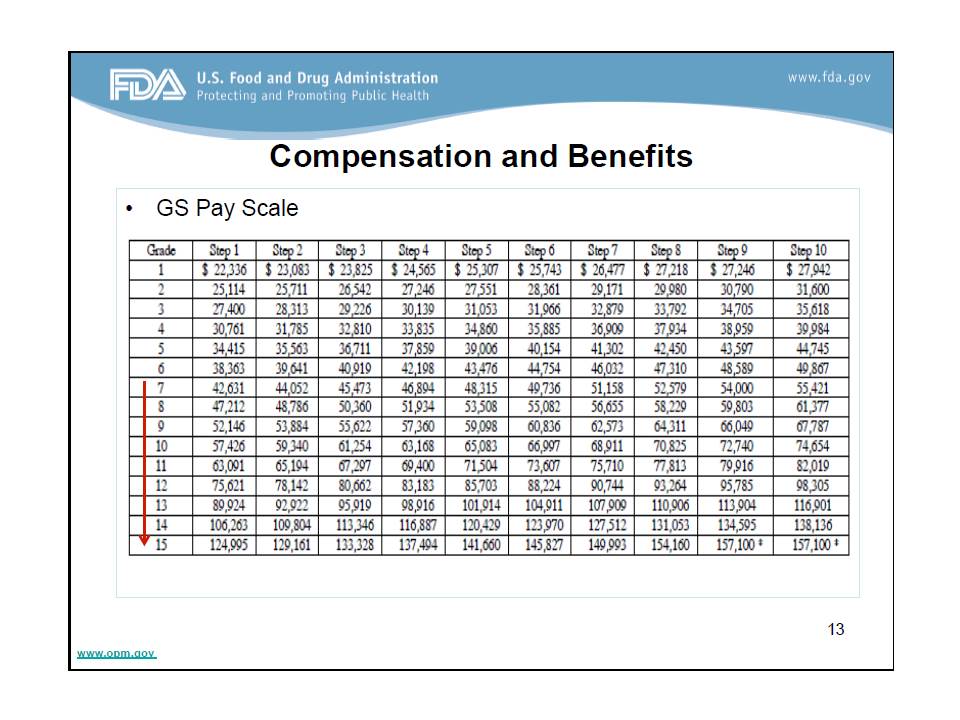
She rounded off her talk by sharing the US government general schedule (GS) pay scale as an overview of the compensation and benefits one would expect working for the FDA (see figure). Researchers’ salaries typically range from grade 7 to 15, depending on educational training and experience. Master’s degree holders typically start at grade 7 as entry level, while Ph.D. graduates can start from grade 13. These values also change based on geographic location due to changes in cost of living. You can learn more about the GS pay scale here.
Dr. Donna J. Green, M.D. is a Medical Officer and a member of the Pediatric Clinical Pharmacology Staff where she is involved in regulatory science research on neonatal and pediatric clinical pharmacology. She obtained her medical degree from the Howard University College of Medicine in D.C. and did clinical training in pediatric medicine at the Herman and Walter Samuelson Children’s Hospital at Sinai in Baltimore. During her training, she became increasingly interested in the rationale behind dosing of children’s medicine, as well as variable drug responses. Because of this, instead of doing a residency and setting up a clinical practice, she completed a clinical pharmacology fellowship at the Drug Discovery Program at the Georgetown University Medical Center. Like Dr. Humeniuk, she then applied and was accepted into the FDA's Commissioner’s Fellowship Program, where she received training on regulatory science, and applied research practices in organ transplantation and pharmacogenomics. Her career path is ideal for MDs, PharmDs, and RNs looking to work in the FDA.
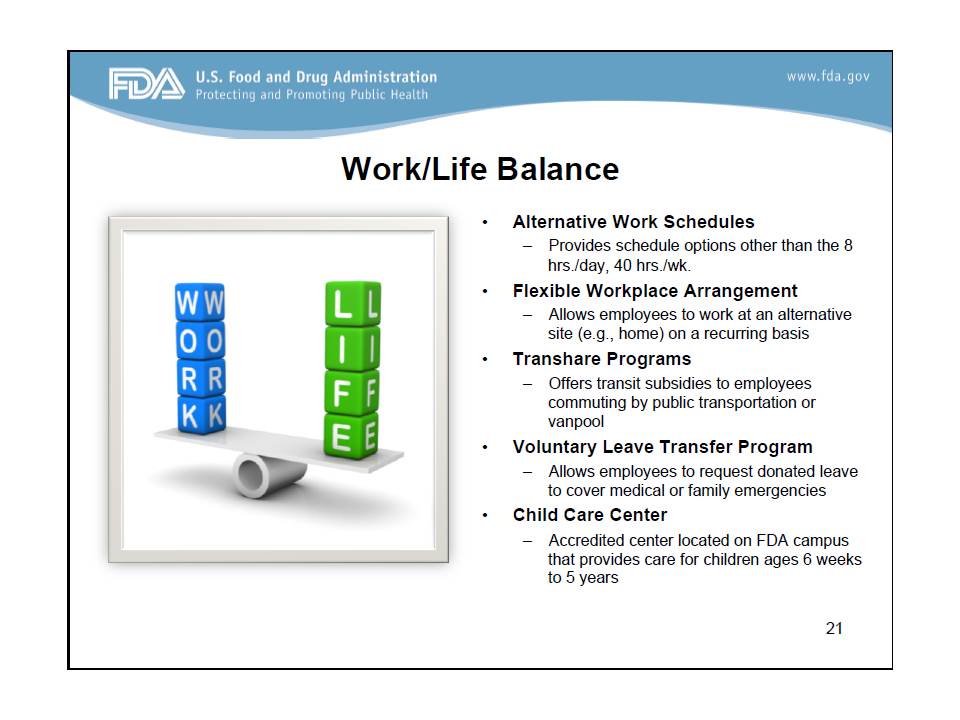
Dr. Green also shared several benefits of working at the FDA, namely 1) excellent work/life balance,
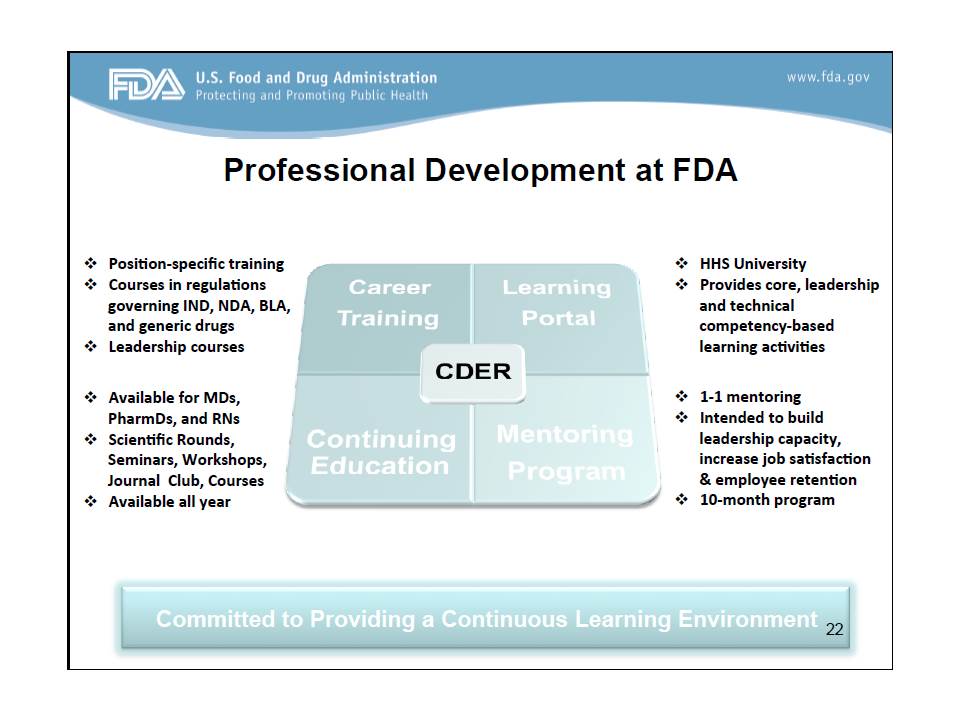
and 2) lots of opportunities for professional development.
Lastly, both speakers presented several fellowship opportunities in the FDA:
- Commissioner’s Fellowship
- 2 year fellowship (15 spots available)
- must have a doctoral level degree
- considered an FDA employee (have benefits in addition to salary)
- open only to green card/US citizens
- ORISE Fellowship
- temporary appointment (limited to a total of 5 years)
- considered a contractor (a monthly stipend but no benefits)
- open to pre-doctoral students, faculty, foreign medical trainees, and summer research participants
- Interagency Oncology Task Force (IOTF) Fellowship
- 2-5 year fellowship
- must have a doctoral level degree
- considered an FDA employee (salary and benefits)
- Regulatory Pharmaceutical Fellowship
- 2 year fellowship
- FDA-Academia-Industry joint fellowship
- Open to PharmD degree holders only
These are only a few of the many opportunities to get involved with the FDA. For a more comprehensive list, visit their site (link).
After their talk, the speakers answered several questions from the audience. First, a Master’s in Public Health, although not required, is a plus if you want to work in the FDA. Next, a postdoc or previous work experience are big pluses and will affect the starting salary grade. Lastly, they mentioned that there is a high turnover between the FDA and industry, and they pointed out that industry experience makes you more competitive in the FDA, and conversely, experience in the FDA makes you more competitive for industry. The speakers’ concluding remarks emphasized that more than a passion for research, people who work at the FDA are passionate about the overall goal of the agency, which is to Promote and Protect Public Health.
Click here for the complete presentation slides and here for a podcast of the talk.