I cannot stress how important informational interviews are in helping you decide your career track. I first learned about regulatory medical writing at an informational interview session. I became so interested that now I am trying to learn more about the career track as a phase 2 iJOBS trainee. Informational interviews give you an opportunity to ask detailed and essential questions about a career and help you learn how to prepare for it. Another great thing about informational interviews is that they can help point you to other career tracks, even ones that you may not have considered. The more interviews I do with scientific professionals, the more career trajectories and great tips/advice I get. Recently, I did a half-day set of interviews at Merck (Rahway, NJ), where I met with six women in various careers. Learning from them was incredibly rewarding!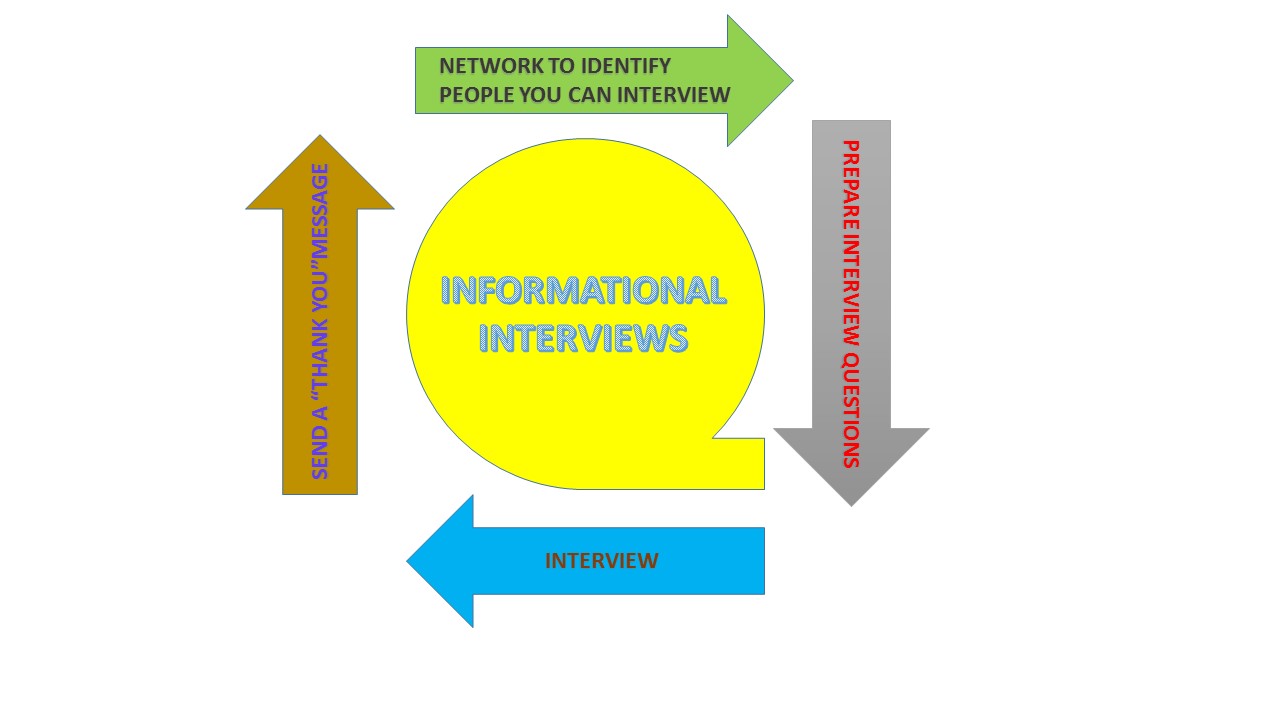 My first meeting was with Cathy Doherty, the program operations lead for clinical operations. Her therapeutic focus is on the bladder. It was exciting to learn that she has been involved with many different products that are now on the market! I was delighted to get in-depth information about her role at Merck and learn about how her work contributes to clinical operations. Her major duties involve project planning for clinical trials and ensuring that the trials run according to plan. She sets a project timeline, helps choose the facilities that are compliant for a clinical trial, deals with queries that arise, and often examines the data from the trials. In the beginning of her career, Cathy loved analyzing data and interacting with people, but was more interested in having a quantifiable impact on human lives. Her bachelor’s degree in Computer Science, Masters in Business Administration and previous job experiences outside of pharma, all helped prepare her for her current responsibilities at Merck. She finds her current career very rewarding because she knows that she continuously helps people live better lives. The field of medical writing, specifically regulatory medical writing, is of interest to me, therefore, I was introduced to Radha Naik-Murti (Ph.D.), the director of medical writing. At the beginning of our meeting she said to me, “loving to write is key to loving a position in regulatory medical writing because it is like writing a thesis every day.” One can infer that knowing how to write effectively and loving the process is essential for a position in this field. What is not so obvious, is that one must also possess excellent reading skills and know how to analyze texts, which are almost as crucial as the writing. Luckily, these are skills that we are already learning as Ph.D. candidates (and enhancing as iJOBS bloggers!). According to Radha, there is no typical day in the life of a regulatory medical writer; each day varies, so being flexible is very important as plans can change at a whim. Interestingly, Radha went through a similar journey as a lot of us. She earned her Ph.D., and then did a postdoc. After these experiences, she was not interested in academia, but could not find a research position in industry, as many companies were struggling at the time. Medical writing was one of the other alternatives she had. She told me, “Becoming a medical writer should not be a way to get your foot in the industry.” What she meant was, if, you are not interested in writing, do not pursue this path. Make sure you consider other options and find what makes you happy. So, if you are considering a career in this field, take her advice into consideration. My conversation with her was an eye-opener and it allowed me to really reflect on what I truly want in a career. The next interview was with my phase 2 iJOBS mentor, Dr. Melissa Tice, who had graciously planned my visit. Our meeting was less of an interview and more of a discussion, as I was already familiar with her background. Dr. Tice studied Chemistry as an undergrad and did her Ph.D. in Neuroscience before transitioning to the NIH for postdoctoral training. She started her career as a researcher at Schering-Plough, which later merged with Merck. Prior to the merge, she made a lateral move and transitioned to regulatory affairs where she is currently an executive director. Some of her responsibilities include managing crises that may arise with clinical trials or health agencies, reading and approving new projects, reviewing and editing documents, developing strategies for drug testing and acceptance, and dealing with drug supply issues. She has spent many years in her current field and often interacts with many of the women I interviewed. To that note, a role in regulatory affairs often allows one to work with a wide range of professionals— from clinical scientists to worldwide product labeling professionals to R&D scientists. In my own job search, she advised me to look at job postings, highlight the job descriptions and determine how I can fit the roles that companies are looking for. She also told me about other career tracks within the industry such as drug advertising and promotion, medical affairs, and licensing and technology. If any of these sounds interesting to you, then you should dig deeper as well! Worldwide product labeling (WPL) is another career opportunity I learned about that day when I spoke with Judyann Wiltsie, who recently transitioned to WPL. Prior to that, she did bench research at Merck. If you work within this field, you are expected to write health-agency authorized drug information that physicians use in prescribing a drug. You also write product labels for drugs approved in other countries, so, much of your time will be spent in meetings with representatives from foreign countries. Product labels can differ in each country because each has their own standards, which may be guided by culture and religion. At Merck, for each drug, three different product labels are written: US format, EU format and one for the Merck headquarters. The last one serves as the basis for all health agency formats, however, it also has all of the collated information about the drug. Once a product label is written, it will be reviewed by regulatory affairs professionals, physicians, clinical scientists, and statisticians, among others. After the necessary changes are approved, the label can then be sent to health authorities for review. It was exciting to find out that there is a high demand for new hires in this field and companies look for candidates with different backgrounds, e.g. research, nursing and pharmacy. At Merck, I spent my lunch time interviewing Tessa Carducci, Ph.D., who does analytical testing at the R&D facility. Although she has been with the company for less than 5 years she is currently the lead for her team. Her current role involves managing projects, collaborating with teams from other groups, writing memos, and training new staff. She spends about half her day in meetings by representing the analytical team and transmitting the information back to her team members. In research, timing is everything, so Tessa sometimes works late and spends weekends in the lab. As she puts it, “having experimental obstacles does not change deadlines in the industry”, so she often finds herself working extra hours. She loves what she does and has a great team of people, which makes the work easier. Her least favorite thing about her job is doing things that have nothing to do with bench laboratory work, meaning administrative and logistical tasks that must be completed The common theme that I took away from the day was that, being flexible is important in industry, and Tessa agreed. If you are interested doing research in the industry, keep in mind that you are already trained for most of the skills (hard and soft) needed. It is your responsibility to know how to use those skills in marketing yourself. My last meeting was with Karni Schlessinger, Ph.D., who also works within regulatory affairs with Dr. Tice. She started her career doing bench work, but found her niche in regulatory affairs. According to her, postdoc experience is almost irrelevant in the industry if you want to stay in research. Karni says this because, despite having a postdoc experience, it did not have an impact on her responsibilities in industry, and did not necessarily put her ahead. This may be because the goals and mindset in industry are very different from those in academia. In her current position as a regulatory liaison, she enjoys coming up with the next disease target for the company. She designs protocols for clinical trials, learns the regulatory rules, and liaises between the company and health agencies. She enjoys researching information and coming up with new ideas, which are skills we learn while obtaining our Ph.D.’s. As someone considering regulatory medical writing for a starting career, she advised me to plan further ahead. According to her, “the width of change might be smaller if you start out in medical writing.” This means that someone who starts out doing research in industry may find it easier to make a lateral change than someone who starts out in medical writing. In other words, your starting position can either widen opportunities or narrow them. So, do not just plan your next step, always plan 2-3 steps ahead. Better yet, determine your ultimate career goal, plan backwards, and start from the career opportunity that gets you to the ultimate goal. In general, the common theme during the interviews was that working in industry requires flexibility. While most of the interviewees can work from home a few times a week, they still have to be able to make changes to their schedule at a whim. Additionally, teamwork is crucial in industry as teams are essential in efficiently moving projects forward. Many of the skills (team work, project management, flexibility etc.) that these pharmaceutical industry professionals have are ones that we also possess. They are skills that will help you in any career trajectory, whether it is in academia or industry. For now, your job is to plan ahead; schedule informational interviews, network and determine your career moves.
My first meeting was with Cathy Doherty, the program operations lead for clinical operations. Her therapeutic focus is on the bladder. It was exciting to learn that she has been involved with many different products that are now on the market! I was delighted to get in-depth information about her role at Merck and learn about how her work contributes to clinical operations. Her major duties involve project planning for clinical trials and ensuring that the trials run according to plan. She sets a project timeline, helps choose the facilities that are compliant for a clinical trial, deals with queries that arise, and often examines the data from the trials. In the beginning of her career, Cathy loved analyzing data and interacting with people, but was more interested in having a quantifiable impact on human lives. Her bachelor’s degree in Computer Science, Masters in Business Administration and previous job experiences outside of pharma, all helped prepare her for her current responsibilities at Merck. She finds her current career very rewarding because she knows that she continuously helps people live better lives. The field of medical writing, specifically regulatory medical writing, is of interest to me, therefore, I was introduced to Radha Naik-Murti (Ph.D.), the director of medical writing. At the beginning of our meeting she said to me, “loving to write is key to loving a position in regulatory medical writing because it is like writing a thesis every day.” One can infer that knowing how to write effectively and loving the process is essential for a position in this field. What is not so obvious, is that one must also possess excellent reading skills and know how to analyze texts, which are almost as crucial as the writing. Luckily, these are skills that we are already learning as Ph.D. candidates (and enhancing as iJOBS bloggers!). According to Radha, there is no typical day in the life of a regulatory medical writer; each day varies, so being flexible is very important as plans can change at a whim. Interestingly, Radha went through a similar journey as a lot of us. She earned her Ph.D., and then did a postdoc. After these experiences, she was not interested in academia, but could not find a research position in industry, as many companies were struggling at the time. Medical writing was one of the other alternatives she had. She told me, “Becoming a medical writer should not be a way to get your foot in the industry.” What she meant was, if, you are not interested in writing, do not pursue this path. Make sure you consider other options and find what makes you happy. So, if you are considering a career in this field, take her advice into consideration. My conversation with her was an eye-opener and it allowed me to really reflect on what I truly want in a career. The next interview was with my phase 2 iJOBS mentor, Dr. Melissa Tice, who had graciously planned my visit. Our meeting was less of an interview and more of a discussion, as I was already familiar with her background. Dr. Tice studied Chemistry as an undergrad and did her Ph.D. in Neuroscience before transitioning to the NIH for postdoctoral training. She started her career as a researcher at Schering-Plough, which later merged with Merck. Prior to the merge, she made a lateral move and transitioned to regulatory affairs where she is currently an executive director. Some of her responsibilities include managing crises that may arise with clinical trials or health agencies, reading and approving new projects, reviewing and editing documents, developing strategies for drug testing and acceptance, and dealing with drug supply issues. She has spent many years in her current field and often interacts with many of the women I interviewed. To that note, a role in regulatory affairs often allows one to work with a wide range of professionals— from clinical scientists to worldwide product labeling professionals to R&D scientists. In my own job search, she advised me to look at job postings, highlight the job descriptions and determine how I can fit the roles that companies are looking for. She also told me about other career tracks within the industry such as drug advertising and promotion, medical affairs, and licensing and technology. If any of these sounds interesting to you, then you should dig deeper as well! Worldwide product labeling (WPL) is another career opportunity I learned about that day when I spoke with Judyann Wiltsie, who recently transitioned to WPL. Prior to that, she did bench research at Merck. If you work within this field, you are expected to write health-agency authorized drug information that physicians use in prescribing a drug. You also write product labels for drugs approved in other countries, so, much of your time will be spent in meetings with representatives from foreign countries. Product labels can differ in each country because each has their own standards, which may be guided by culture and religion. At Merck, for each drug, three different product labels are written: US format, EU format and one for the Merck headquarters. The last one serves as the basis for all health agency formats, however, it also has all of the collated information about the drug. Once a product label is written, it will be reviewed by regulatory affairs professionals, physicians, clinical scientists, and statisticians, among others. After the necessary changes are approved, the label can then be sent to health authorities for review. It was exciting to find out that there is a high demand for new hires in this field and companies look for candidates with different backgrounds, e.g. research, nursing and pharmacy. At Merck, I spent my lunch time interviewing Tessa Carducci, Ph.D., who does analytical testing at the R&D facility. Although she has been with the company for less than 5 years she is currently the lead for her team. Her current role involves managing projects, collaborating with teams from other groups, writing memos, and training new staff. She spends about half her day in meetings by representing the analytical team and transmitting the information back to her team members. In research, timing is everything, so Tessa sometimes works late and spends weekends in the lab. As she puts it, “having experimental obstacles does not change deadlines in the industry”, so she often finds herself working extra hours. She loves what she does and has a great team of people, which makes the work easier. Her least favorite thing about her job is doing things that have nothing to do with bench laboratory work, meaning administrative and logistical tasks that must be completed The common theme that I took away from the day was that, being flexible is important in industry, and Tessa agreed. If you are interested doing research in the industry, keep in mind that you are already trained for most of the skills (hard and soft) needed. It is your responsibility to know how to use those skills in marketing yourself. My last meeting was with Karni Schlessinger, Ph.D., who also works within regulatory affairs with Dr. Tice. She started her career doing bench work, but found her niche in regulatory affairs. According to her, postdoc experience is almost irrelevant in the industry if you want to stay in research. Karni says this because, despite having a postdoc experience, it did not have an impact on her responsibilities in industry, and did not necessarily put her ahead. This may be because the goals and mindset in industry are very different from those in academia. In her current position as a regulatory liaison, she enjoys coming up with the next disease target for the company. She designs protocols for clinical trials, learns the regulatory rules, and liaises between the company and health agencies. She enjoys researching information and coming up with new ideas, which are skills we learn while obtaining our Ph.D.’s. As someone considering regulatory medical writing for a starting career, she advised me to plan further ahead. According to her, “the width of change might be smaller if you start out in medical writing.” This means that someone who starts out doing research in industry may find it easier to make a lateral change than someone who starts out in medical writing. In other words, your starting position can either widen opportunities or narrow them. So, do not just plan your next step, always plan 2-3 steps ahead. Better yet, determine your ultimate career goal, plan backwards, and start from the career opportunity that gets you to the ultimate goal. In general, the common theme during the interviews was that working in industry requires flexibility. While most of the interviewees can work from home a few times a week, they still have to be able to make changes to their schedule at a whim. Additionally, teamwork is crucial in industry as teams are essential in efficiently moving projects forward. Many of the skills (team work, project management, flexibility etc.) that these pharmaceutical industry professionals have are ones that we also possess. They are skills that will help you in any career trajectory, whether it is in academia or industry. For now, your job is to plan ahead; schedule informational interviews, network and determine your career moves.
iJOBS Blog