iJOBS Career Fair: What you Can Do with a Ph.D.
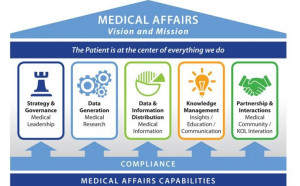
 The annual iJOBS/BioNJ career fair is the epitome of what you can do with a Ph.D. The iJOBS program stresses that Ph.D.’s can do much more than practice science in the traditional sense. The opportunities are essentially boundless.
The annual iJOBS/BioNJ career fair is the epitome of what you can do with a Ph.D. The iJOBS program stresses that Ph.D.’s can do much more than practice science in the traditional sense. The opportunities are essentially boundless.