By Antonia Kaz
Artificial intelligence (AI) has changed how we perform daily tasks like deciding what to eat for dinner, how to politely decline a work-related request while on vacation and learn. Thus, it is no surprise that the AI model ChatGPT has had the highest adoption rate among online technologies introduced in the 21st century. Netscribes reported in 2023 that within two months, ChatGPT hit 100 million users while apps like TikTok and Instagram took 9 months and 2.5 years, respectively. As a society, we quickly learned how to use AI to enhance our daily lives, but scientists have been pushing the boundaries of what AI models can do. They are using the rapidly evolving AI models and open access tools to answer the questions our doctors need an answer to: Will this drug work for my patient? Dr. Brandon Higgs, the Global Head of Translational Data Science at the international biotech company Genmab, is a bioinformatics scientist and computational biologist using AI technology to support translational scientists involved in determining patient prognoses, identifying patients with favorable subtypes for a therapy, and the development of effective drug combination therapies. His most recent iJOBS and Erdos Institute Seminar defined AI buzz words, explained how this technology is used at Genmab, and provided advice for young scientists interested in incorporating this technology into their research.
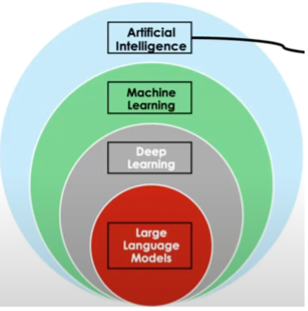
AI is a branch of computer science that uses machines or computers to perform a task that humans would normally perform. The concept of computers thinking like humans was first proposed by Alan Turing in 1950. He developed a method to test this called the “Turing test”. This paved the way for current AI models.
Machine learning enables computers with algorithms to learn information and recognize patterns or predict outcomes. Various social media apps and music apps have adopted this AI model to provide more personalized content for their users. Deep learning uses deep neural networks on large ustructured datasets to predict outcomes. In 2020, the chemistry Nobel was awarded to Drs. David Baker, Demis Hassabis, and John Jumper for a revolutionary deep learning approach to predict the structure of proteins directly from its sequence, AlphaFold. AlphaFold 2 is free and openly available through their database. Genmab has used deep learning neural networks to characterize and quantify different cells within the tissue contexture, such as lymphocytes, red blood cells, tumor, etc., of patient biopsies that are taken in nearly every cancer screening. Dr. Higgs emphasized this technology would not replace pathologists because there still needs to be “ground truth” and a professional to check the work. Instead, it would minimize the burden on pathologists. The continued development of this technology enabled deep learning models to identify an immunogenic phenotype within tumors, a technology which could help decrease the number of biopsies taken in cancer patients. This is important because there are current drug therapies capable of improving patient prognosis if they have a specific tumor subtype.
Large language models (LLMs) are the central topic of AI technologies used in drug discovery. LLMs modes use natural language to predict and generate text that responds to questions and summarizes information. The underlying premise of these models is to provide a probability of predicting the next word from the input data. LLMs are developed with two basic concepts in mind: how words are related based on the documents provided and how words are related based on the sentence or context.
More refined answers have been generated using a Retrieval Augmented Generation approach (RAG) that uses the input to retrieve a set of relevant documents. This approach reduces the number of hallucinations or artifacts from the responses. Genmab used generative AI technology to predict successful drug combinations for a specific tumor type using data from preclinical and clinical trials and provide a rationale behind the predictions. Dr. Higgs described how the initial trials using data assessing the efficacy of drug combinations in clinical trials resulted in predictions based on patterns of when drugs were used together rather than with new combinations. Thus, they needed to train the model about various drug therapies using a knowledge graph and include preclinical studies that provide quantitative measures of the efficacy of drug combinations to be able to predict new combinations. This resulted in a new prediction for a combination therapy to treat platinum-resistant ovarian cancer and evidence to support their efficacy based on their mechanisms of action and targets within the tumor type. Dr. Higgs explained how this predicition lacked a confidence interval and reasons why the prediction may not work.
All hypotheses, whether computer-generated or from leading scientists in a field, must be tested through rigorous experimental designs to determine the efficacy and toxicity of novel drug therapeutics. Generative AI technology should be used as a tool, complementing what scientists already know by encouraging creative new therapeutic strategies that can be tested in preclinical studies. If you are interested in using AI in your research or working in the bioinformatics industry, Dr. Higgs recommended a few ways to begin introducing AI technology into your work. Start by asking large language models like ChatGPT or Gemini questions that may come to you after a seminar or meeting. If you are a seasoned AI user, repositories of AI models are readily available on the internet, and you can download and learn how to use them with the help of YouTube videos. Getting familiar with these innovative technologies early on will help build and train systems to benefit the research we do as scientists. If you are interested in Genmab's work with AI, learn more here.
This article was edited by Junior Editor Janaina Cruz Pereira and Senior Editor Joycelyn Radeny.